Instruções aos autores
INSTRUÇÕES AOS AUTORES – REVISTA MINEIRA DE ENFERMAGEM (REME)
Os manuscritos submetidos à REME devem atender aos requisitos da sua ''Política Editorial'' e das
normas de publicação descritas nas ''Instruções aos Autores''. Leia atentamente todas as instruções antes da submissão. O não cumprimento implicará atraso na avaliação.
1. SOBRE A REVISTA E ESCOPO
A Revista Mineira de Enfermagem (REME) publica manuscritos originais e inéditos que contribuam
para o conhecimento e desenvolvimento da Enfermagem e áreas afins. São aceitos manuscritos nas
seguintes seções:
- Editorial
- Pesquisa (Artigo original)
- Revisão (metanálises, revisões sistemáticas e de escopo)
- Relato de experiência ou de caso
- Reflexão
A composição dos volumes prioriza a tipologia Pesquisa (80%) e reserva 20% para os demais tipos.
A publicação é bilíngue: português/inglês ou espanhol/inglês. Os manuscritos podem ser enviados
em português, inglês ou espanhol, com resumo no idioma original do manuscrito. A versão do
resumo para o inglês (Abstract) será de responsabilidade dos tradutores/revisores contratados pelos
autores; a versão para o espanhol (Resumen) é de responsabilidade da REME, sendo elaborados após
a aprovação por revisores/tradutores credenciados.
Os manuscritos devem ser apresentados em conformidade com os requisitos estabelecidos nestas instruções, elaboradas em consonância às normas do International Committee of Medical Journal Editors (ICMJE), ''Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals'' e do ''Uniform Requirements for Manuscripts - URM'' -, que segue as normas ''Citing Medicine'' - The NLM Style Guide for Authors, Editors and Publishers - da ''National Library of Medicine -NLM'' adotado pelo ICMJE.
Estas normas estão disponíveis na íntegra nos endereços:
Em português: http://www.bu.ufsc.br/ccsm/vancouver.html
Em espanhol: http://www.enfermeriaencardiologia.com/formacion/vancouver.htm
Em inglês: http://www.nlm.nih.gov/bsd/uniform_requirements.html
2. TAXAS
- Taxa de avaliação: R$ 150,00 (paga após aceite na pré-análise)
- Taxa de publicação: R$ 750,00 (após aprovação final)
Ambas são cobradas via boleto bancário emitido pela FUNDEP/UFMG (Projeto 4828*1). Não há reembolso de valores pagos indevidamente ou em caso de rejeição.
3. TIPOS DE MANUSCRITOS E LIMITES
O número de palavras e referências é limitado para os diversos tipos de artigos, conforme indicado no quadro a seguir. No quantitativo de palavras está incluído o corpo do texto, agradecimentos, ilustrações; não estão incluídos na contagem de palavras o resumo e as referências. Para as ilustrações (gráficos, gravuras, fotografias, mapas, esquemas, desenhos, tabelas, quadros, fórmulas, modelos e outros) indica se o máximo de cinco, independentemente do tipo.
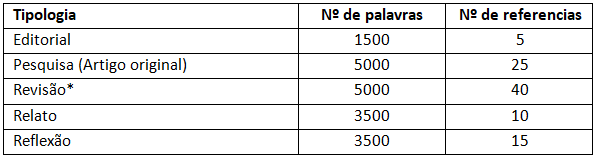
*Informamos que estão suspensas, temporariamente, as submissões de revisões narrativas e integrativas. São aceitas revisões sistemáticas, metanálises e revisões de escopo.
4. DIRETRIZES METODOLÓGICAS
Os manuscritos submetidos à apreciação da REME devem ser reportados conforme as diretrizes e guias da EQUATOR Network (https://www.equator-network.org/reporting-guidelines/) e das Recomendações do ICMJE (https://www.icmje.org/recommendations/) na preparação dos manuscritos. Faça o download, preencha o checklist e anexe na submissão.
Alguns dos guias para os principais tipos de estudos são:
- Ensaios clínicos: CONSORT https://www.equator-network.org/reporting-guidelines/consort/.
- Estudos observacionais em epidemiologia: STROBE https://www.equator-network.org/reporting-guidelines/strobe/. Traduções:
- Revisões sistemáticas/ escopo: PRISMA https://www.equator-network.org/reporting-guidelines/prisma/
- Protocolos: SPIRIT (protocolo de ensaios clínicos) https://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/ ou PRISMA-P (protocolo de revisão) https://www.equator- network.org/reporting-guidelines/prisma-protocols/
- Estudos de acurácia diagnóstica: STARD https://www.equator-network.org/reporting-
guidelines/stard/
- Relatos de caso: CARE https://www.equator-network.org/reporting-guidelines/care/
- Pesquisa qualitativa: COREQ: https://www.equator-network.org/reporting-guidelines/coreq/
Checklists traduzidos para diversos idiomas podem ser acessados em: https://www.equator- network.org/post_type=eq_guidelines&eq_guidelines_study_design=0&eq_guidelines_clinical_ pecialty=0&eq_guidelines_report_section=0&s=+CONSORT+extension&btn_submit=Searc +Reporting+Guidelines
5. REQUISITOS ÉTICOS E REGISTRO
- Comitê de Ética: Em conformidade com as normas constantes nas Resoluções nº 466/2012 e nº 510/2016 do CNS/BR. Quando a pesquisa envolver seres humanos, deve obrigatoriamente informar aprovação por um Comitê de Ética em Pesquisa e o número do protocolo (Certificado de Apresentação para Apreciação Ética – CAAE e/ou número do parecer de aprovação) na seção de Métodos. Anexar o documento no sistema de submissão.
- Ensaio clínico: deve ser registrado antes da coleta de dados e aprovado por uma das entidades descritas a abaixo e o número do registro deve constar no resumo e anexado no sistema. Mais informações sobre registros de ensaios clínicos estão disponíveis em: http://www.icmje.org/about-icmje/faqs/clinical-trials-registration/
o Australian New Zealand Clinical Trials Registry (ANZCTR)
o ClinicalTrials.gov
o International Standard Randomised Controlled Trial Number (ISRCTN)
o UMIN Clinical Trials Registry (UMIN-CTR)
o WHO International Clinical Trials Registry Platform (ICTRP)
o Registro Brasileiro de Ensaios Clínicos (ReBEC)
- Fonte de financiamento: declarar todas as fontes de financiamento ou de apoio institucional público e/ou privado. Quando não houver, os autores devem declarar que a pesquisa não recebeu financiamento para sua realização.
- Conflito de interesse: declarar a existência ou inexistência.
6. PREPARAÇÃO DO MANUSCRITO
- Editor de texto: Word (.doc/.docx). Não serão aceitos artigos em formato PDF. Faça o download do template de submissão e insira seu texto conforme as instruções no arquivo.
- Fonte: Times New Roman, 12 pt, espaçamento simples (Resumo, contribuições dos autores e referências: fonte 10 pt, espaçamento simples).
- Citações diretas com mais de 3 linhas/falas de entrevistados: fonte 10 pt, espaçamento simples, recuo de 4 cm.
Estrutura do texto do manuscrito:
1. Indicação da seção (Editorial, Pesquisa, Revisão, Relato, Reflexão)
2. Título no idioma original do manuscrito. Deve ser conciso e informativo, com até 15 palavras. A identificação do tipo do estudo no título, quando obrigatória, não entrará na contagem de palavras.
3. Resumo (Abstract ou Resumen) (até 200 palavras) no idioma original do manuscrito. Para os artigos de ''Pesquisa'', o resumo deve ser apresentado no formato estruturado segundo as seções do manuscrito: objetivos, método, resultados e conclusão (pesquisas quantitativas) ou considerações finais (pesquisas qualitativas). A versão do resumo para o inglês (abstract) será de responsabilidade dos tradutores/revisores contratados pelos autores; a versão para o espanhol (resumen) é de responsabilidade da REME.
4. Palavras-chave, keywords e palabras clave (de três a seis), devem ser indicadas de acordo com o ''Descritores em Ciências da Saúde'' (DECS) da BIREME, Centro Latino-Americano e do Caribe de Informação em Ciências da Saúde, disponível em: http://decs.bvs.br/, tradução do Medical Subject Headings (MESH) da National Library of Medicine NLM/NIH, disponível em: http://www.ncbi.nlm.nih.gov/mesh. As palavras-chave devem ser apresentadas em ordem alfabética.
5. Corpo do texto: A estrutura do manuscrito nas categorias “Pesquisa” e “Revisão” é: Introdução, Métodos, Resultados, Discussão e Conclusões (para pesquisa quantitativa) ou Considerações finais (pesquisa qualitativa).
6. Agradecimentos (opcional)
7. Referências
7. APRESENTAÇÃO DE FIGURAS E TABELAS
As figuras e tabelas (no máximo cinco), devem ser apresentadas em formato aberto/editável (Word, Excel, Powerpoint etc.). Todas as tabelas e figuras deverão ser mencionadas no texto. Exemplo: “...conforme apresentado na Figura 1...”.
Figuras
São consideradas figuras: gráficos, gravuras, fotografias, mapas, esquemas, desenhos, quadros, fórmulas, modelos. Todos estes itens devem ser denominados apenas como “Figura” e numerados consecutivamente na ordem em que são mencionados no texto (Exemplo: Figura 1, Figura 2, etc.). Quando extraídos de outras publicações, exige a indicação da fonte.
Os quadros deverão conter dados textuais e não numéricos, ser fechados nas laterais e com linhas
internas, fonte com tamanho mínimo de 10 pt, espaçamento simples. Por exemplo:
Figura 1 - Normas utilizadas na formatação de trabalhos acadêmicos
![]()
Fonte: Elaborado pelos autores.
Tabelas
As tabelas devem conter título informativo, claro e completo, localizado acima do seu conteúdo. Deve constar no título das tabelas o local (cidade, sigla do estado, país), ano da coleta de dados e o “n” (tamanho da amostra). As tabelas deverão conter dados numéricos, ser abertas nas laterais e sem linhas internas, fonte com tamanho mínimo de 10 pt, espaçamento simples. Utilizar linhas
superior, inferior e abaixo do cabeçalho somente. Apresente dados que se complementam em coluna única, por exemplo frequência absoluta e relativa “n (%)”. Padronize em todo o texto e nas tabelas a nomenclatura “valor-p” para se referir ao valor de p. Padronize o número de casas decimais utilizados após a vírgula. Por exemplo:
Tabela 1 – Distribuição dos participantes segundo sexo, faixa etária e escolaridade. Belo Horizonte, MG, Brasil, 2024 (n = 150)
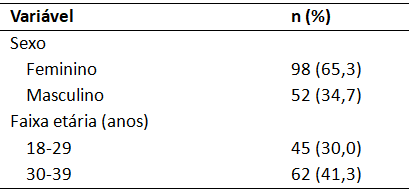
Fonte: Dados da pesquisa.
Tabela 2 – Associação entre variáveis independentes e presença de hipertensão arterial em adultos. Belo Horizonte, MG, Brasil, 2024 (n = 320)
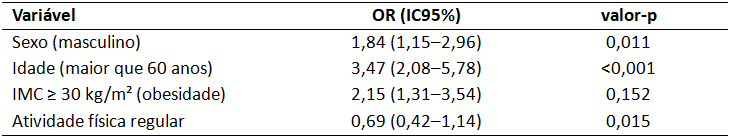
Legenda: OR – razão de chances; IC95% – intervalo de confiança de 95%.
Fonte: Dados da pesquisa.
Para mais informações, consultar Normas de apresentação tabular: IBGE, 3ª ed., 1993. Disponível em:https://biblioteca.ibge.gov.br/visualizacao/livros/liv23907.pdf
8. CITAÇÕES E REFERÊNCIAS
- Citações indiretas no texto: números arábicos, sobrescritos entre parênteses, antes do ponto, sem espaço entre texto e citação, correspondendo às referências indicadas no final do artigo. Quando forem sequenciais, indicar o primeiro e o último número, separados por hífen. Ex.: (1-4); quando intercaladas, deverão ser separadas por vírgula, sem espaço. Ex.: (1-5,8) ou (3,7,18). Por exemplo:
(...) 20% apresentaram desempenho moderado(5).
Conforme observado por Silva et al.(3), a grande maioria...
O mesmo padrão já foi verificado em estudos anteriores(1,2,5-8).
- O Formato adotado para as referências bibliográficas é o “ Estilo Vancouver”- Citing Medicine - The NLM Style (https://www.ncbi.nlm.nih.gov/books/NBK7256/).
- As referências são numeradas consecutivamente, na ordem em que são mencionadas/citadas pela primeira vez no texto. Documentos com até seis autores, listar todos; mais de seis autores, listar os seis primeiros seguido de ‘et al.’ Disponibilizar link (de preferência o link do DOI) e data de acesso sempre que houver.
- Os títulos dos periódicos são abreviados conforme o NLM Catalog Journals (PubMed) (https://www.ncbi.nlm.nih.gov/nlmcatalog/journals), ou com o Portal de Revistas Científicas em Saúde da BVS (Bireme/OPAS/OMS) (http://portal.revistas.bvs.br/).
Exemplos mais comuns de referências:
Artigos de periódicos
Kim MS, Park JH, Park KY. Development and Effectiveness of a Drug Dosage Calculation Training Program using Cognitive Loading Theory based on Smartphone Application. J Korean Acad Nurs [Internet]. 2012 [cited 2022 Mar 12];42(5):689-98. Available from: https://doi.org/10.4040/jkan.2012.42.5.689
Sousa MLA, Shimizu IS, Patino CM, Torres-Duque CA, Zabert, I, Zabert GE, et al. Conhecimento, atitudes e práticas em relação à COVID-19 entre profissionais de saúde na América Latina. J Bras Pneumol [Internet]. 2022 [citado em 2022 mar. 12];48(5):e20220018. Disponível em: https://doi.org/10.36416/1806-3756/e20220018
Mantovani VM, Nazareth JK, Maciel DNP, Biasibetti C, Lucena AF, Echer IC. Absenteísmo por enfermidade em profissionais de enfermagem. REME Rev Min Enferm. [Internet]. 2015 [citado em 2025 jun. 1];19(3):641-6. Disponível em: https://periodicos.ufmg.br/index.php/reme/article/view/50073
Livros
Potter PA, Perry AG, Stockert PQ, Hall AM. Fundamentos de Enfermagem. 11ª ed. Rio de Janeiro: GEN Guanabara Koogan; 2024.
Maried E, Hoehn K. Human Anatomy & Physiology. 11th ed. London: Pearson; 2018. 1264 p.
Livro na Internet/Ebook
Ministério da Saúde. Estratégia nacional para promoção do aleitamento materno e alimentação complementar saudável no Sistema Único de Saúde. Brasília (DF): Ministério da Saúde, 2015 [citado em 2023 out. 16]. 152 p. Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/estrategia_nacional_promocao_aleitamento_materno.pdf
Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions [Internet]. Version 4.2.6. Chichester (UK): John Wiley & Sons, Ltd.; 2006 [cited 2018 Oct15]. 257 p. Available from: http://www.cochrane.org/resources/handbook/handbook.pdf
Capítulo de livro de autoria diferente da autoria do livro no todo
Bachega K, Accetturi E. Transplantes de tecido ósseos no Brasil: uma história segura de sucesso da odontologia. In: Santos PS, Mello WR, Coracin FL, Balnda RC, organizators. Odontologia em transplante de órgãos e tecidos. Curitiba: Editora CRV; 2018. p. 109-27.
Capítulo de livro de mesma autoria do livro no todo
Borges EL, Saar SRC, Magalhães MBB, Gomes FSL, Lima VLAN. Feridas: como tratar. 2ª ed. Belo Horizonte (MG): Editora Médica COOPMED; 2010. Capítulo 10, Custo do tratamento de feridas; p. 179-88.
Teses e dissertações
Silva, KL. Promoção da saúde em espaços sociais da vida cotidiana [tese de doutorado]. Belo Horizonte (MG): Universidade Federal de Minas Gerais; 2009 [citado em 2023 maio 12]. 182 p. Disponível em: http://hdl.handle.net/1843/GCPA-7T6JVK
Documentos Legislativos
Constituição Brasileira
Brasil. [Constituição (1988)]. Constituição da República Federativa do Brasil [Internet]. Brasília, DF: Senado Federal; 2016 [cited 2019 Mar 19]. 496 p. Available from: https://www2.senado.leg.br/ bdsf/bitstream/handle/id/518231/CF88_Livro_EC91_2016.pdf
Lei e Decretos
Brasil. Lei nº 13.709, de 14 de agosto de 2018. Dispõe sobre a proteção de dados pessoais e altera a Lei nº 12.965, de 23 de abril de 2014 (Marco Civil da Internet). Diário Oficial da União. 2018 Aug 15;155(157 seção 1):59-64.
Resoluções
Conselho Federal de Medicina. Resolução CFM nº 2.180/2018. Estabelece os dados de médicos que devem ser disponibilizados em consultas eletrônicas relacionadas aos registros dos profissionais médicos inscritos no Sistema Conselhos de Medicina e dá outras providências. Diário Oficial da União. 2018 Sept 19;155(181 seção 1):128.
Portarias
Fundação de Amparo à Pesquisa do Estado de São Paulo. Portaria PR nº08/2015. Dispõe sobre prorrogação de bolsas em razão do advento de prole [Internet]. São Paulo: FAPESP; 2015 [cited 2019 Mar 19]. Available from: http://www.fapesp.br/9593
9. INFORMAÇÕES ADICIONAIS
Se o artigo derivar de tese ou dissertação, essa informação deve ser indicada.
As abreviaturas, grandezas, símbolos e unidades devem observar as Normas Internacionais de Publicação. Ao empregar pela primeira vez uma abreviatura, esta deve ser precedida do termo ou expressão completa, salvo quando se tratar de uma unidade de medida comum.
As medidas de comprimento, altura, peso e volume devem ser expressas em unidades do sistema métrico decimal (metro, quilo, litro) ou seus múltiplos e submúltiplos; as temperaturas, em graus Celsius; os valores de pressão arterial, em milímetros de mercúrio.
Agradecimentos devem constar de parágrafo à parte, inserido antes das referências.
10. SUBMISSÃO NO OJS
A submissão dos manuscritos é realizada no site da REME pelo Sistema OJS, na aba "Submissão Online".
Instruções para submissão de manuscritos no sistema também estão disponíveis no tutorial "Processo de Submissão", disponível em: https://www.youtube.com/watch?v=Pus_57HpwTU.
| Documento | Obrigatoriedade | Como utilizar/enviar |
|
Arquivo do manuscrito, sem identificação dos autores (clique para baixar o template) |
Todos |
Enviar conforme normas para preparação do manuscrito. |
|
Página de título (clique para baixar o modelo) |
Todos |
Fazer o download do modelo, preencher com as informações dos autores e enviar durante a submissão. |
|
Declaração de responsabilidade e transferência de direitos autorais e contribuições dos autores (clique para baixar) |
Todos |
Fazer o download do documento, preenchê-lo e enviá-lo durante a submissão, assinado por todos os autores. |
|
Aprovação do Comitê de Ética em Pesquisa |
Para estudos com seres humanos |
Enviar em formato PDF durante a submissão. |
|
Registro do ensaio clínico |
Para ensaios clínicos |
|
|
Checklist (clique para baixar) e fluxograma CONSORT (clique para baixar) |
Para ensaios clínicos |
Fazer o download dos documentos, utilizá-los na preparação do artigo, preenchê-los e enviá-los durante a submissão. |
|
Checklist (clique para baixar) e fluxograma PRISMA (clique para baixar) |
Para revisões sistemáticas e metanálises |
|
|
Checklist e fluxograma PRISMA-ScR (clique para baixar) |
Para revisões de escopo |
|
|
Checklist COREQ (clique para baixar) |
Para estudos qualitativos |
|
|
Checklist STROBE (clique para baixar) |
Para estudos observacionais |
|
|
Declaração de Uso de Inteligência Artificial (clique para baixar) |
Todos |
Fazer o download do documento, preenchê-lo e enviá-lo durante a submissão assinado por todos os autores. |
11. TRADUÇÕES E REVISÕES LINGUÍSTICAS
- Manuscritos submetidos em português ou espanhol: o autor providenciará a tradução para o inglês (abstract e texto completo) após aprovação.
- Manuscrito submetido em inglês: o autor providenciará a versão em português.
- A tradução do resumo para o espanhol é de responsabilidade da REME.
- A revisão deve ser feita por tradutores/revisores credenciados pela REME.
12. AUTORIA
Todas as informações de autoria devem ser colocadas na Página de título disponível para download, e não no arquivo principal do manuscrito. Este não deve conter dados que permitam a identificação dos autores.
a) Limite: 8 autores por artigo.
b) Nomes e sobrenomes dos autores
Todos os autores deverão ser incluídos diretamente no sistema de submissão OJS no ato de submissão. Não serão incluídos autores a posteriori. Indicação do nome(s) completo(s) do(s) autor(es) no primeiro campo e no último campo o sobrenome sem preposições como “e”, “da”, “de”, “do” etc, independentemente do idioma em que o documento estiver escrito, no segundo campo indicado. Indicar o nome completo, sem abreviaturas. Não incluir pronomes de tratamento nem nomes intermediários.
c) Autor correspondente
Nome completo e endereço eletrônico do autor responsável para correspondência.
d) ORCID
É obrigatório o cadastro do autor no Open Researcher and Contributor ID (ORCID) e inserção, através do link enviado por e-mail, no sistema de submissão OJS. O campo ORCID (no cadastro de autores) deve ser preenchido com o endereço completo: https://orcid.org/0000-0000-0000-0000.
e) Instâncias institucionais (afiliação) e geográficas
Indicação da(s) instituição(ões) de afiliação de cada autor e sua localização geográfica, em até três níveis hierárquicos, do maior para o menor, seguido da localização: cidade, estado e país.
Exemplo:
Universidade Federal de Minas Gerais - UFMG, Escola de Enfermagem - EE, Departamento de Enfermagem Básica - ENB. Belo Horizonte, MG - Brasil.
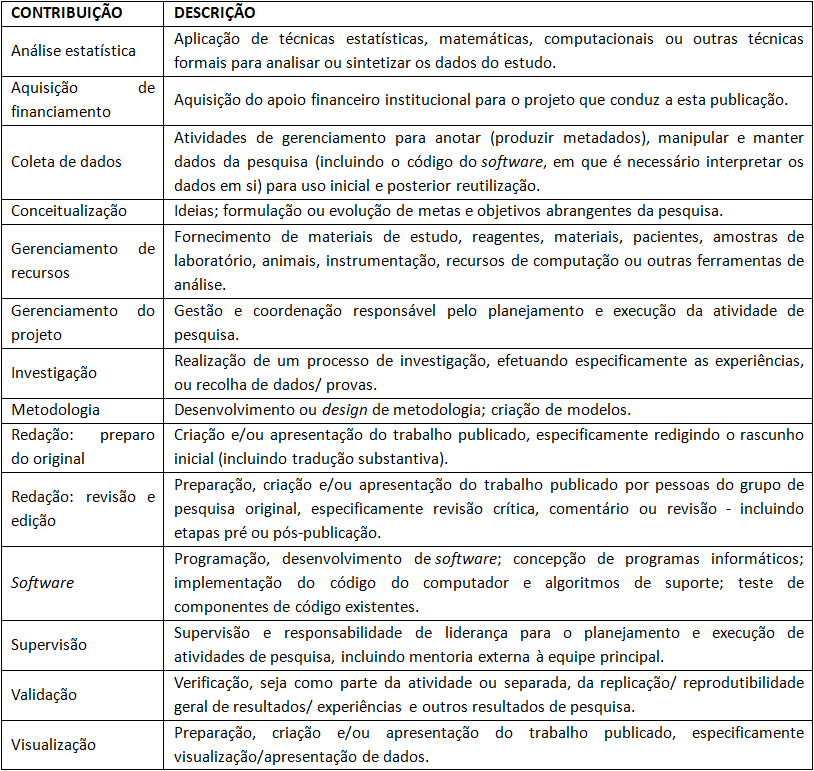
f) Contribuições de autoria
Cada autor deve declarar sua participação conforme o modelo do ICMJE. As categorias incluem:
13. ETAPAS PÓS-APROVAÇÃO
- Revisão da normalização e padronização
- Revisão editorial e linguística
- Atribuição de DOI
- Publicação do ahead of print
- Tradução (conforme idioma de envio)
- Diagramação e aprovação final dos PDFs
- Publicação em português e inglês (PDF e HTML)
Divulgação em:
- Sites: REME, EEUFMG e COREN-MG.
- Portais: Periódicos CAPES, Periódicos da UFMG, REV@ENF.
- Redes sociais (Facebook, Instagram)
14. DIREITOS AUTORAIS E RESPONSABILIDADES
- Os direitos autorais são transferidos à REME no ato da submissão.
- A REME não se responsabiliza pelas opiniões expressas nos artigos.
- Os casos omissos são resolvidos pelo Conselho Editorial.
15. CONDUTAS ÉTICAS E PLÁGIO
As questões acerca de conflitos de interesses, aspectos éticos e os procedimentos adotados quando são evidenciadas más condutas em manuscritos submetidos estão descritos na POLÍTICA EDITORIAL. REME utiliza os softwares "CheckForPlagiarism.Net" e "Ithenticate" para verificação de similaridade.
Em caso de dúvidas consulte o FAQ no site ou envie e-mail à secretaria da revista.